Journal of Investigative Dermatology: Interleukin 1 Receptor–Associated Kinase 4 is Overexpressed in Hidradenitis Suppurativa Skin and Correlates with Inflammatory Biomarkers
Journal of Investigative Dermatology: Interleukin 1 Receptor–Associated Kinase 4 is Overexpressed in Hidradenitis Suppurativa Skin and Correlates with Inflammatory Biomarkers
Alice McDonald, Rahul Karnik, Veronica Campbell, Jeff Davis, Sara Chavoshi, Anthony Slavin, Kirti Sharma, Jared Gollob, Afsaneh Alavi
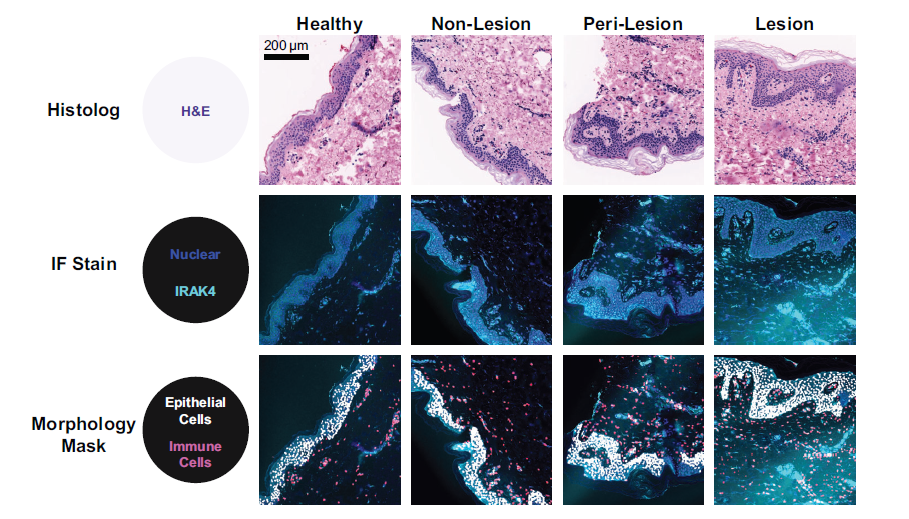
Hidradenitis suppurativa (HS) is a chronic inflammatory disease manifesting as painful dermal nodules, abscesses, and tunnels. Activation of the IL-1R/toll-like receptor (TLR) pathway is strongly implicated in the pathogenesis of HS; thus, the role of a key signaling node, IL-1R–associated kinase 4 (IRAK4), was investigated in a noninterventional study (NCT04440410) that enrolled 30 patients with HS. IRAK4 expression was evaluated in blood and lesional, perilesional, and nonlesional skin biopsies. Peripheral blood mononuclear cells (PBMCs) expressed IRAK4, with significantly higher levels in monocytes (P ≤ 0.0001). Ex vivo treatment of PBMCs with KT-474, a targeted degrader of IRAK4, robustly decreased IRAK4 in all immune cell types from healthy volunteers and patients with HS. Ex vivo treatment of TLR-stimulated healthy donor monocytes with KT-474 decreased IRAK4 protein levels and inhibited inflammatory cytokine production. In HS skin samples, IRAK4 protein levels were significantly higher in lesional versus nonlesional tissue (P ≤ 0.0001), and IRAK4-positive immune infiltrate increased with greater disease severity. Multiple inflammatory mediators were upregulated in HS lesional skin, correlating with IRAK4 overexpression. These data confirm the significance of the IL-1R/TLR pathway in the pathogenesis of HS and provide support for ongoing clinical studies evaluating KT-474 in the treatment of HS.