Pioneering Targeted Protein Degradation to Invent New Medicines
Targeted protein degradation (TPD) is a powerful drug modality that harnesses the cell’s existing protein disposal and recycling machinery to eradicate disease-causing proteins that cannot be addressed, or are poorly addressed, by other technologies, delivering a potentially transformative new way to fight disease and dramatically improve patients’ lives.
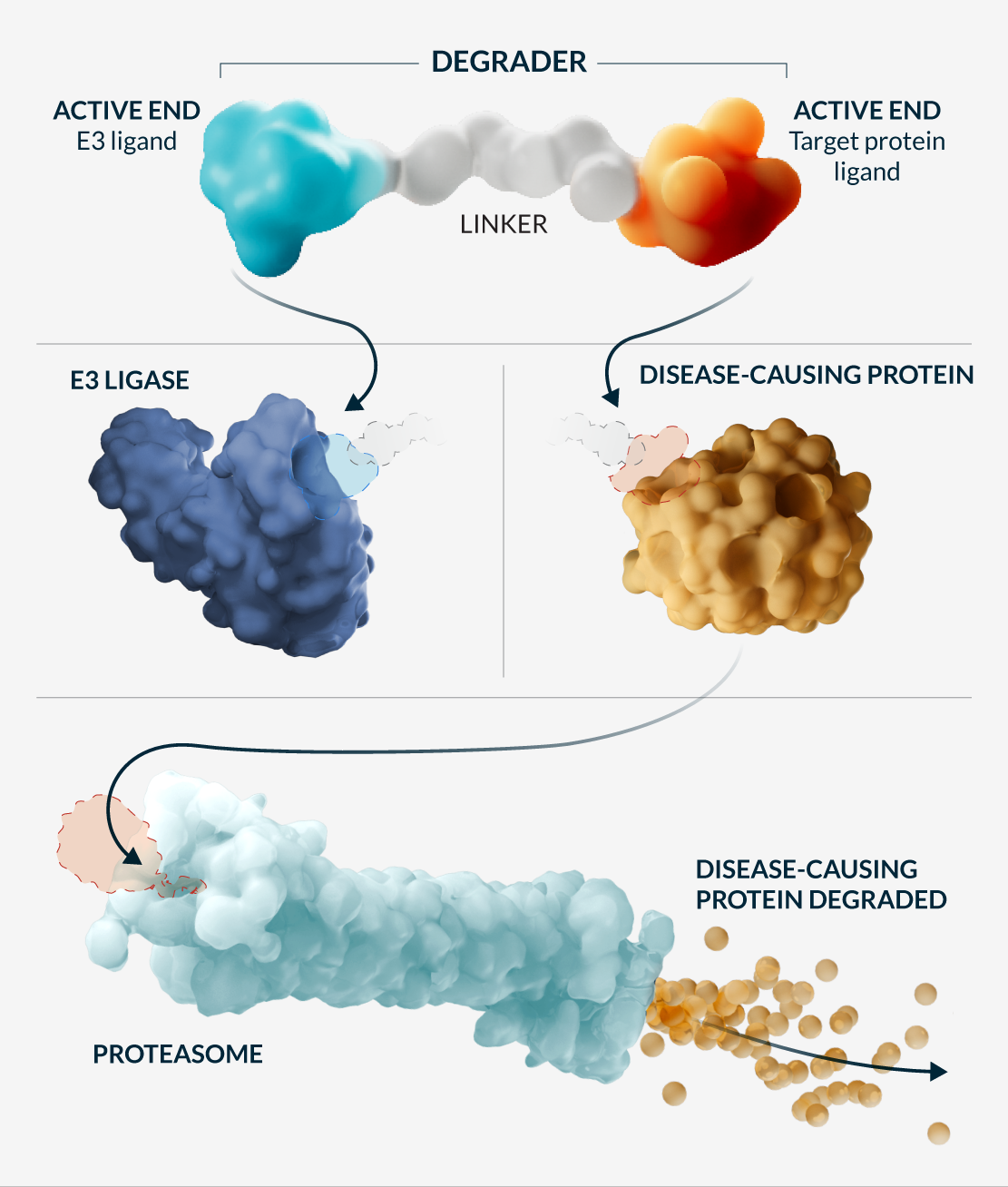
Cells naturally clear out misfolded or accumulated proteins; at Kymera we create heterobifunctional small molecules that redirect this natural process by engaging enzymes called E3 ubiquitin ligases to recruit disease-causing proteins and place a chain of ubiquitin molecules on the recruited protein. The ubiquitin tag is recognized by the cell’s recycling systems—the proteasome—to selectively break down the protein. These molecules are catalytic—they can perform this tagging action many times within the cell.
Better Degraders Built from the Ground Up
Kymera’s differentiated approach is being employed to build a pipeline of first-in-class programs and propel the field of protein degradation forward.
We are focused on deploying our technology against disease targets in areas of significant patient need that can’t be meaningfully addressed by traditional medicines, and where we believe TPD is the only or the best way to elucidate the desired biology or clinical outcome. This approach includes addressing undrugged proteins and broad pathways and nodes with strong genetic and clinical validation in a disease-agnostic way, initially focused on immunology as well as some areas of oncology.
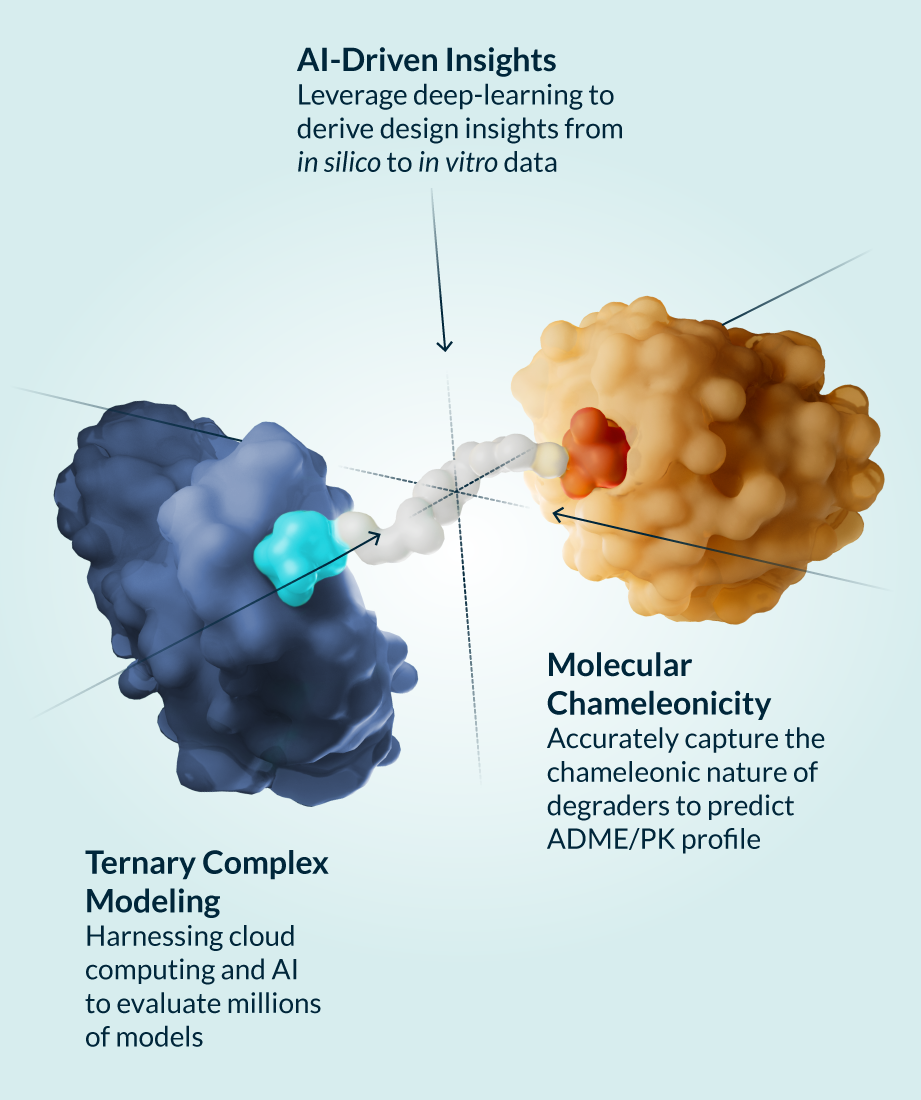
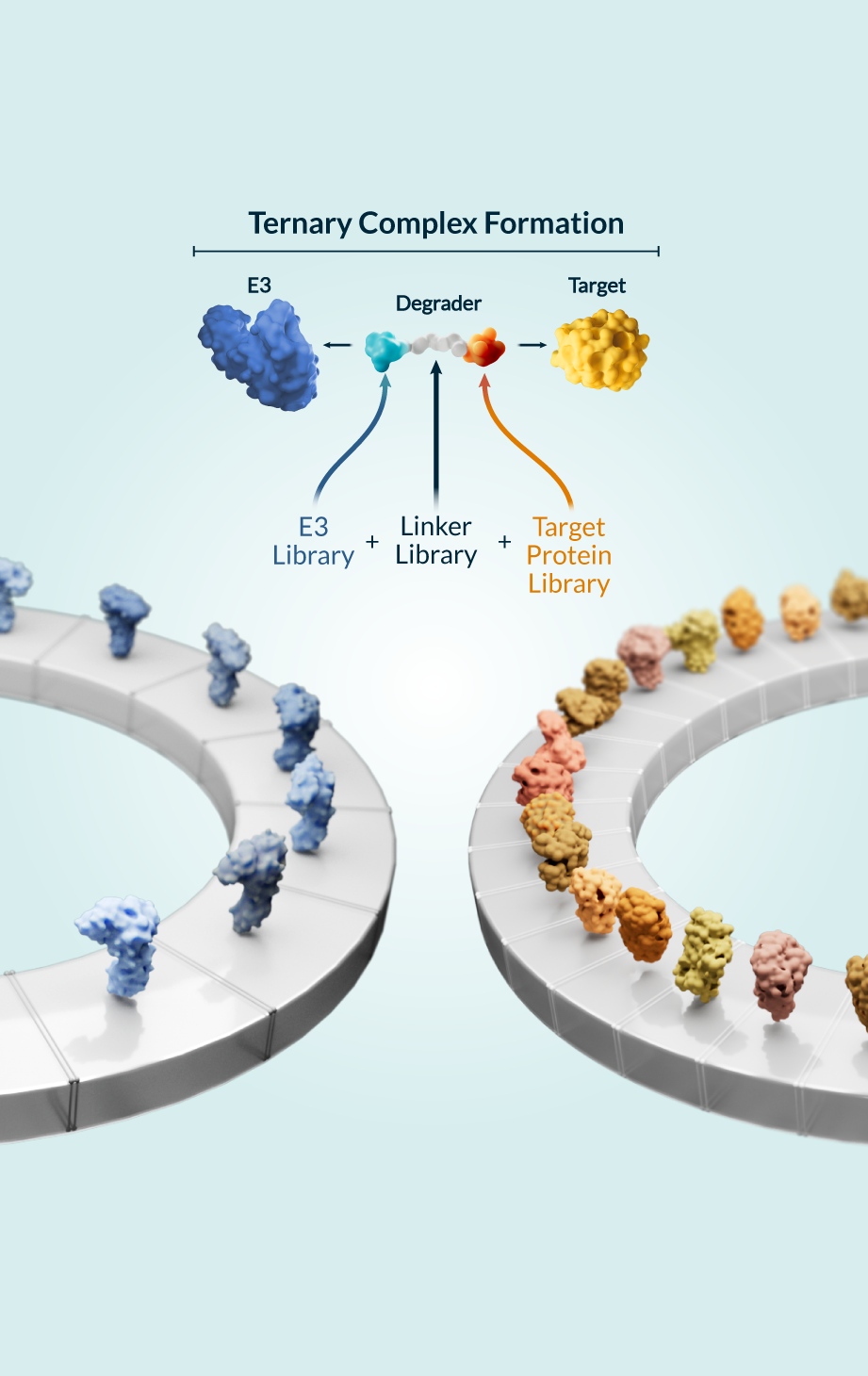
Kymera’s powerful drug discovery engine delivers first-in-class investigational drugs in areas with high unmet need that are primed for clinical-stage development. Developing degrader medicines involves optimizing the design and interactions between components of the modality’s unique ternary complex (the E3 ligase, degrader molecule, and protein to be degraded) to enable the body to eliminate disease-causing proteins. We’ve created a toolbox of integrated approaches to accelerate the discovery and development of transformative degrader medicines, enabling us to study molecular structures and gain a full understanding of the biological mechanisms of proteins including:
- A comprehensive hit identification strategy using high-content as well as fit-for-purpose technologies such as DNA encoded libraries and fragment and X-ray based screenings
- Machine learning and artificial intelligence hit-validation and optimization technologies
- Structure (e.g., X-ray crystallography and cryogenic electron microscopy) and pharmacokinetic/pharmacodynamic-centric enabled lead optimization
- State of the art translational biology focused on patient disease samples
In addition, we work to maximize the efficacy of each molecule by using global quantitative proteomics techniques to match target proteins with the optimal E3 ligase from our library of over 600 unique options across different tissues and cell types.
Our rigorous preclinical work not only sets up our own molecules for clinical success, but is also pushing the entire field forward by shedding light on the best ways to design degraders with optimized properties tailored not only to specific diseases, but also potentially targeted patient populations. We have generated and validated predictive models that reflect our deep knowledge and understanding of the interplay between targets and drug properties, driving optimization of drug disposition and in vitro and in vivo pharmacokinetic/pharmacodynamic relationships of our degraders across different tissue and cell types. We have demonstrated high fidelity of translation in the clinic for all the programs that we have advanced in clinical stage to date. We are also exploring and leading the development of adjacent technologies and next generation TPD strategies to further expand the range of targets for degrader therapies.