Kymera’s pipeline is focused on addressing disease targets where there is significant patient need and where we believe protein degradation is the only or best way to improve the standard of care.
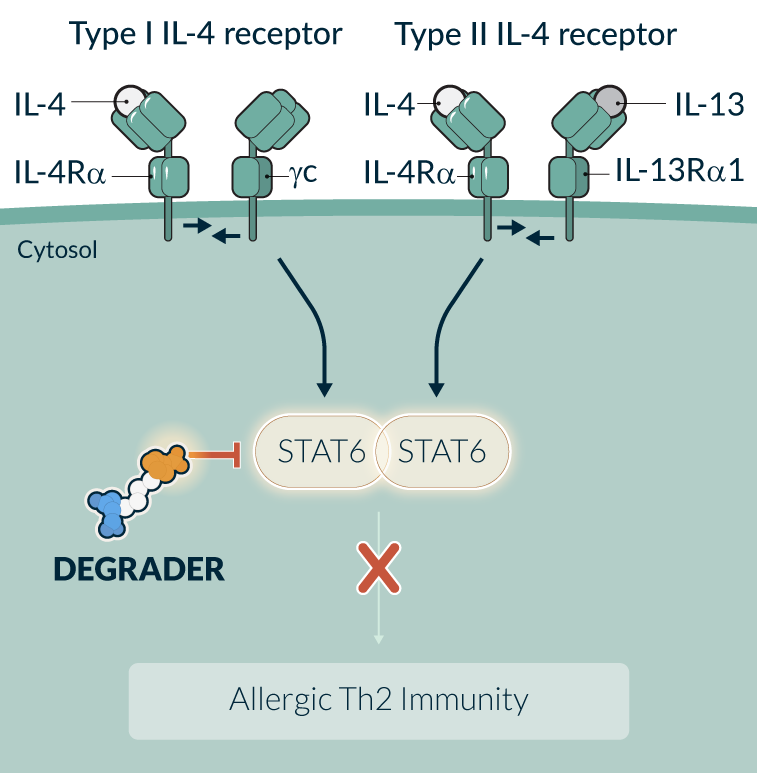
STAT6 is an essential transcription factor in the IL-4/IL-13 signaling pathways and the central driver of TH2 inflammation in allergic diseases. Multiple gain of function mutations of STAT6 were identified to cause severe allergic diseases in humans. Dupilumab, an injectable monoclonal antibody that blocks IL-4/IL-13 signaling, is an approved therapy for multiple allergic diseases. STAT6 targeting is therefore supported by both human genetics and clinical pathway validation. STAT6 functions through protein-protein and protein-DNA interactions and it has been challenging to selectively and potently inhibit STAT6 with small molecule inhibitors. However, it is well suited for a targeted protein degradation approach, where a binding event is sufficient to drive degradation. In preclinical studies, KT-621, Kymera’s first-in-class oral STAT6 degrader, demonstrated full inhibition of the IL-4/IL-13 pathway in all relevant human cell contexts with picomolar potency that was superior to dupilumab, and equivalent or superior efficacy to dupilumab in multiple preclinical efficacy studies. In addition, at low oral doses, KT-621 demonstrated near full in vivo STAT6 degradation and was well-tolerated in multiple preclinical toxicity studies.
Atopic Dermatitis
Asthma
Chronic Obstructive Pulmonary Disease
Chronic Rhinosinusitis with Nasal Polyps
Eosinophilic Esophagitis
Prurigo Nodularis
Others
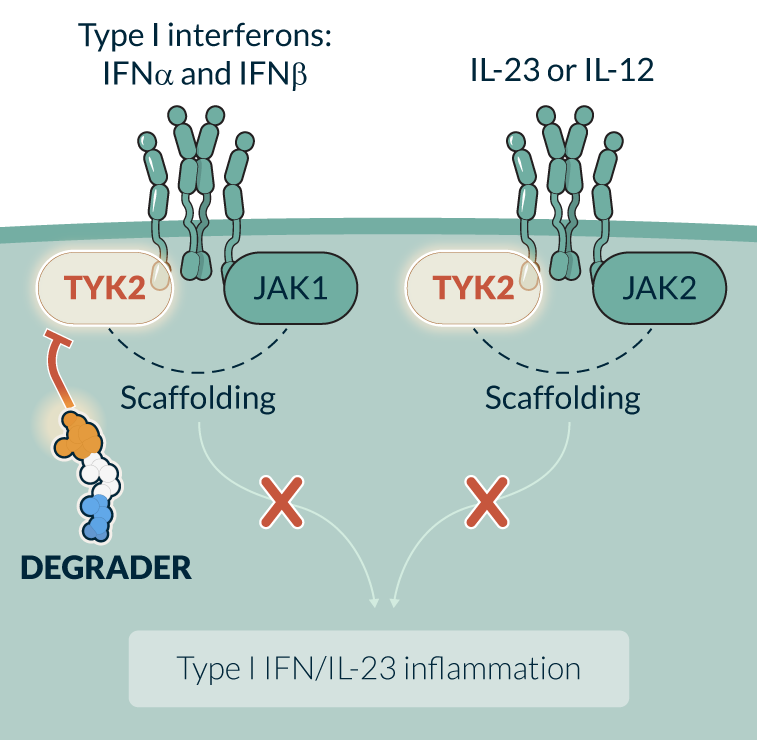
TYK2 is a member of the JAK family of kinases that binds the IL-12, IL-23 and interferon (IFN) receptors to recruit and phosphorylate STAT transcription factors. A loss of function variant is protective in autoimmune diseases and an allosteric inhibitor of TYK2, as well as biological agents targeting IL-12, IL-23 and Type I IFN, have been approved for the treatment of multiple autoimmune diseases, making TYK2 a highly validated target. TYK2 has a well-established scaffolding function that plays a key role in cytokine receptor surface expression and activation. In preclinical studies, KT-295, Kymera’s first-in-class oral TYK2 degrader, has demonstrated picomolar degradation potency and potent inhibition of the IL-23, IL-12 and Type I IFN pathways while sparing IL-10, showing its potential to recapitulate the biology of human TYK2 loss-of-function mutations. Degradation of TYK2 has the potential to overcome the challenges of small molecule inhibitors, which have limitations due to lack of selectivity, limited target engagement, and/or lack of potent activity against Type I IFN.
Inflammatory Bowel Disease
Psoriasis
Psoriatic Arthritis
Lupus
Others
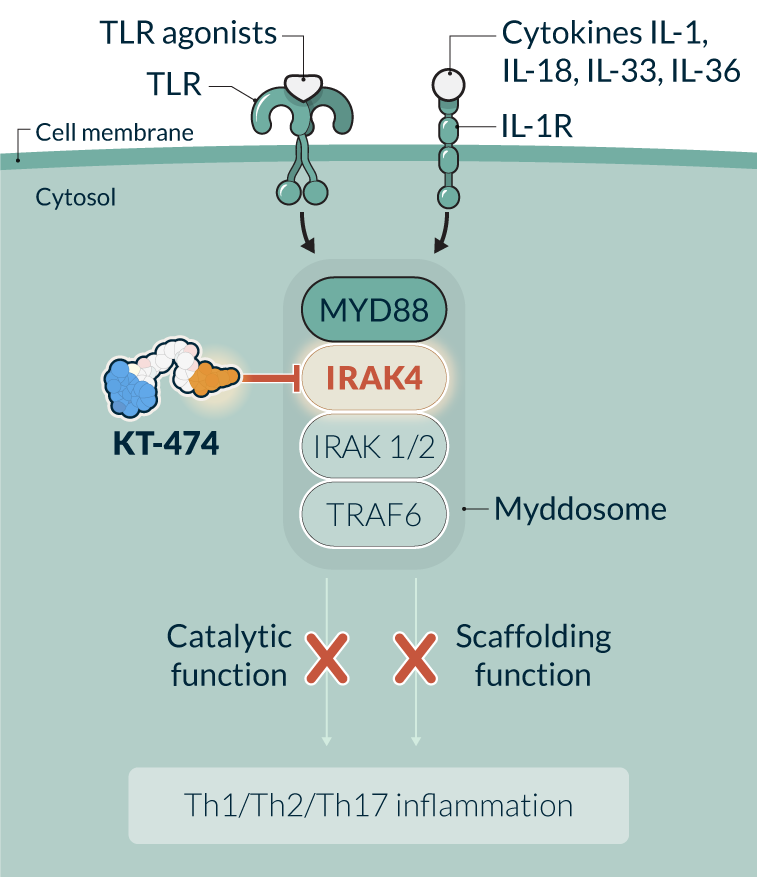
KT-474 is a first-in-class IRAK4 degrader in development for the treatment of immune-inflammatory diseases with significant patient need, such as hidradenitis suppurativa (HS), atopic dermatitis (AD), and potentially others. IRAK4 is a key protein of the myddosome complex that mediates signaling through IL-1 and toll-like receptors, which play a crucial role in initiating the immune response against invading pathogens. IRAK4 is a scaffolding kinase that acts at the interface of the innate and adaptive immune responses with a variety of functions depending on its kinase activity and scaffolding function. Eliminating IRAK4 completely through degradation impacts both the kinase and scaffolding functions, therefore having the potential to achieve a broad, well-tolerated, anti-inflammatory effect providing a novel therapeutic approach for a variety of immune-inflammatory diseases.
KT-474 (SAR444656) is partnered with Sanofi, who is leading the Phase 2 clinical development program. Kymera has the option to participate in future development and commercialization, and 50/50 profit split, in the United States. Double digit tiered royalties in ROW.
Diseases where the TLR/IL-1 pathway has been implicated in pathogenesis and are potential opportunities: Psoriasis, Generalized Pustular Psoriasis, IBD, Asthma, COPD, SSc ILD, RA, PSA, SLE, OA, PSS, MS, AMD.
When appropriate, we partner with leading global pharmaceutical companies to maximize the reach of our degrader programs and develop transformative therapies for the broadest possible patient populations.
Our current partnership with Sanofi is designed to accelerate the path to broader clinical development and commercialization of our first-in-class protein degrader therapies targeting IRAK4 in patients with immune-inflammatory diseases.