
Breakthrough Medicines
Catalyzed by Protein Degradation




Kymera is building a fully integrated medicines company to redefine disease treatment paradigms. Come work with us to shape the future of medicine.

Kymera’s team is catalyzing progress in the science of TPD with key publications and presentations that unlock insights and showcase our findings.
Zhang, Y., Loh, C., Chen, J., & Mainolfi, N.
Zhang, Y., Loh, C., Chen, J., & Mainolfi, N.
Zhang, Y., Loh, C., Chen, J., & Mainolfi, N.

A Powerful Strategy that Puts Patients First
We tackle challenges and push the envelope on behalf of patients every day, pursuing new approaches to revolutionize disease treatment.

We push boundaries with an incredible sense of urgency, knowing patients are waiting for our therapies and that every week, every day, and every hour counts. We tackle challenges such as traditionally “undruggable” disease-causing proteins, pursuing new approaches to revolutionize disease treatment and find solutions for patients.






Just like chimeric molecules combine two or more components to create a powerful and innovative therapeutic opportunity, together, we are greater than the sum of our parts.
We welcome like-minded pioneers to join us on our extraordinary journey as we create a culture where diverse perspectives are invited, and collaboration is celebrated—all in service of our goal to revolutionize medicine and change patients’ lives.
Just like chimeric molecules combine two or more components to create a powerful and innovative therapeutic opportunity, together, we are greater than the sum of our parts.
We welcome like-minded pioneers to join us on our extraordinary journey as we create a culture where diverse perspectives are invited, and collaboration is celebrated—all in service of our goal to revolutionize medicine and change patients’ lives.







Targeted protein degradation is a disease-agnostic technology fundamentally changing our understanding of how drugs need to work and the diseases it’s possible to treat.
This is the m-default-editor.
Sed posuere consectetur est at lobortis. Etiam porta sem malesuada magna mollis euismod. Cras justo odio, dapibus ac facilisis in, egestas eget quam. Morbi leo risus, porta ac consectetur ac, vestibulum at eros. Aenean eu leo quam. Pellentesque ornare sem lacinia quam venenatis vestibulum.
Vivamus sagittis lacus vel augue laoreet rutrum faucibus dolor auctor. Integer posuere erat a ante venenatis dapibus posuere velit aliquet. Duis mollis, est non commodo luctus, nisi erat porttitor ligula, eget lacinia odio sem nec elit. Integer posuere erat a ante venenatis dapibus posuere velit aliquet. Nulla vitae elit libero, a pharetra augue. Nullam quis risus eget urna mollis ornare vel eu leo.
Praesent commodo cursus magna, vel scelerisque nisl consectetur et. Cras mattis consectetur purus sit amet fermentum. Donec id elit non mi porta gravida at eget metus. Nullam quis risus eget urna mollis ornare vel eu leo. Cras mattis consectetur purus sit amet fermentum. Praesent commodo cursus magna, vel scelerisque nisl consectetur et. Praesent commodo cursus magna, vel scelerisque nisl consectetur et. Maecenas faucibus mollis interdum. Donec id elit non mi porta gravida at eget metus. Etiam porta sem malesuada magna mollis euismod. Nullam quis risus eget urna mollis ornare vel eu leo. Vestibulum id ligula porta felis euismod semper. Vestibulum id ligula porta felis euismod semper.
Pioneering Targeted Protein Degradation to Invent New Medicines
Targeted protein degradation (TPD) is a compelling modality that harnesses the cell’s existing machinery to identify and eradicate disease-causing proteins that cannot be addressed, or are poorly addressed, by other modalities, delivering a potentially transformative new way to fight disease and dramatically improve patients’ lives.
Nulla vitae elit libero, a pharetra augue. Morbi leo risus, porta ac consectetur ac, vestibulum at eros. Nullam id dolor id nibh ultricies vehicula ut id elit. Vestibulum id ligula porta felis euismod semper.
Since our founding, we’ve built a preeminent discovery engine capable of identifying new drug candidates at an accelerated pace and delivering at least one new program every year, and we’re advancing a pipeline of high-value programs poised to change patient lives.
We partner with leading global pharmaceutical companies to develop transformative therapies for the broadest possible patient populations.
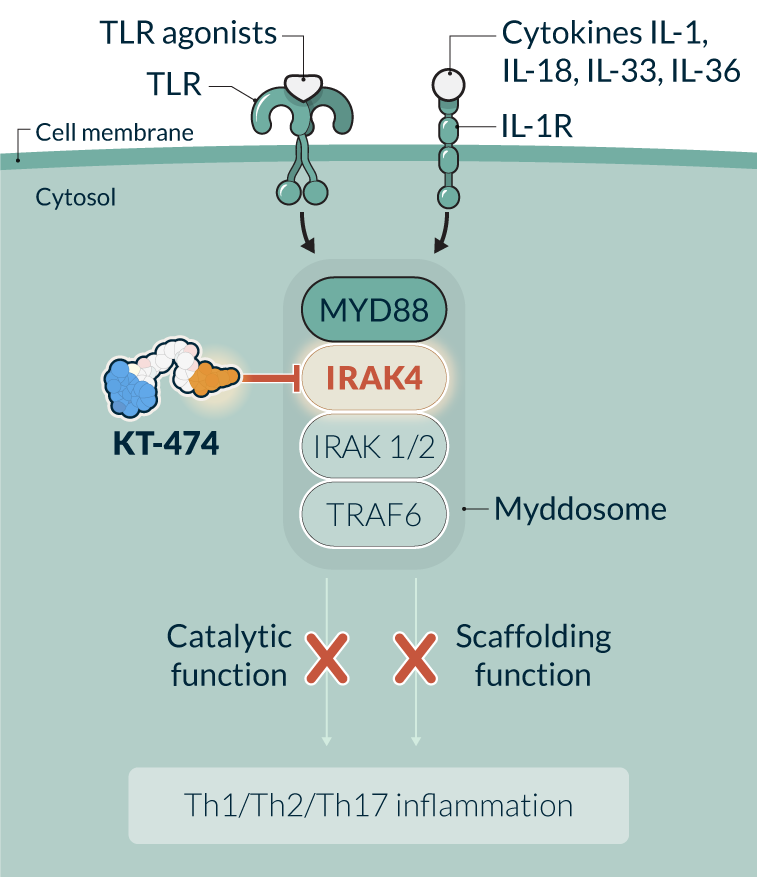
Our current partnership with Sanofi is enabling us to accelerate the path to broader clinical development and commercialization of our first-in-class protein degrader therapies targeting IRAK4 in patients with immune-inflammatory diseases.
Our current partnership with Sanofi is enabling us to accelerate the path to broader clinical development and commercialization of our first-in-class protein degrader therapies targeting IRAK4 in patients with immune-inflammatory diseases.
We’re looking for pioneers to join us on our journey to revolutionize medicine. If you embrace the opportunity to do things that haven’t been done before and want to help us build a company that aspires to change patient’s lives, we may be a good fit for you. You belong at Kymera if you are:
Driven
You don’t shy away from challenges and are compelled by cutting-edge science with the potential to impact patients’ lives.
Community-Oriented
You seek out meaningful connections, collaborations, and friendships in your work environment.
Authentic
You want to bring your authentic self to work and achieve your full potential.
We partner with leading global pharmaceutical companies to develop transformative therapies for the broadest possible patient populations.
Better Degraders Built from the Ground Up
Kymera’s differentiated approach is being employed to build a pipeline of first-in-class programs and propel the field of protein degradation forward.
We are focused on deploying our technology against disease targets in areas of significant patient need that can’t be meaningfully addressed by traditional medicines and where TPD is the only or the best way to elucidate the desired biology or clinical outcome. This approach includes tackling undruggable proteins and addressing broad pathways and nodes with strong genetic validation in immunology and oncology.
Kymera’s powerful drug discovery engine generates high-potential programs that are primed for clinical stage development. Designing degrader medicines involves engaging the components of the modality’s unique ternary complex formation (the E3 ligase, degrader molecule, and protein to be degraded) to enable the body to eliminate disease-causing proteins. We’ve created a toolbox of integrated approaches to accelerate the discovery and development of transformative degrader medicines, and utilize a comprehensive hit identification strategy of biophysical, biochemical, and biological methods, including high throughput screenings and cutting-edge technologies like cryogenic electron microscopy and X-ray crystallography. These capabilities enable us to study molecular structures and gain a full understanding of the biological mechanisms of proteins. In addition, we work to maximize the efficacy of each molecule by using quantitative proteomics techniques to match target proteins with the optimal E3 ligase from our library of over 600 unique options across different tissues and cell types.
Our rigorous preclinical work not only sets up our own molecules for clinical success, but is pushing the entire field forward by shedding light on the best ways to design degraders with optimized properties tailored not only to specific diseases, but also potentially targeted patient populations. We have generated and validated predictive models that reflect our deep knowledge and understanding of the interplay between targets and drug properties, driving optimization of drug disposition and in vitro and in vivo pharmacokinetic/pharmacodynamic relationships of our degraders across different tissue and cell types. We are also exploring and leading the development of adjacent technologies and next generation TPD strategies to further expand the range of targets for degrader therapies.
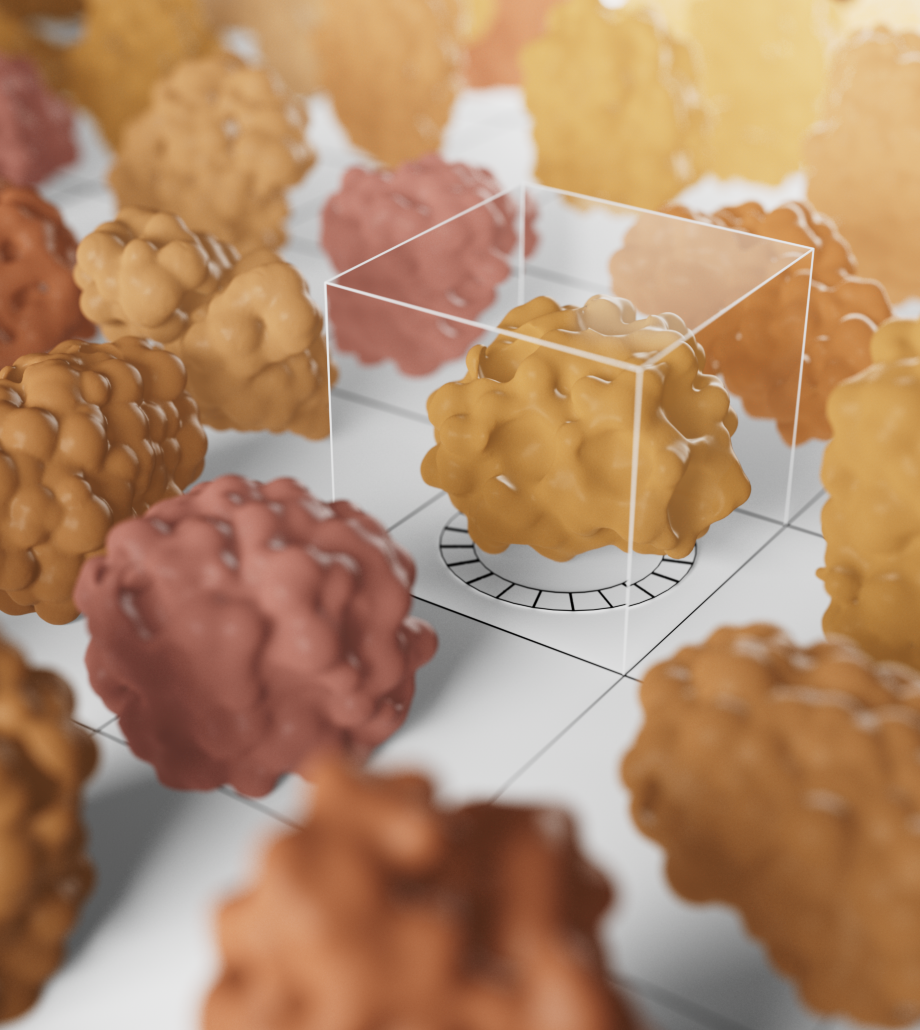
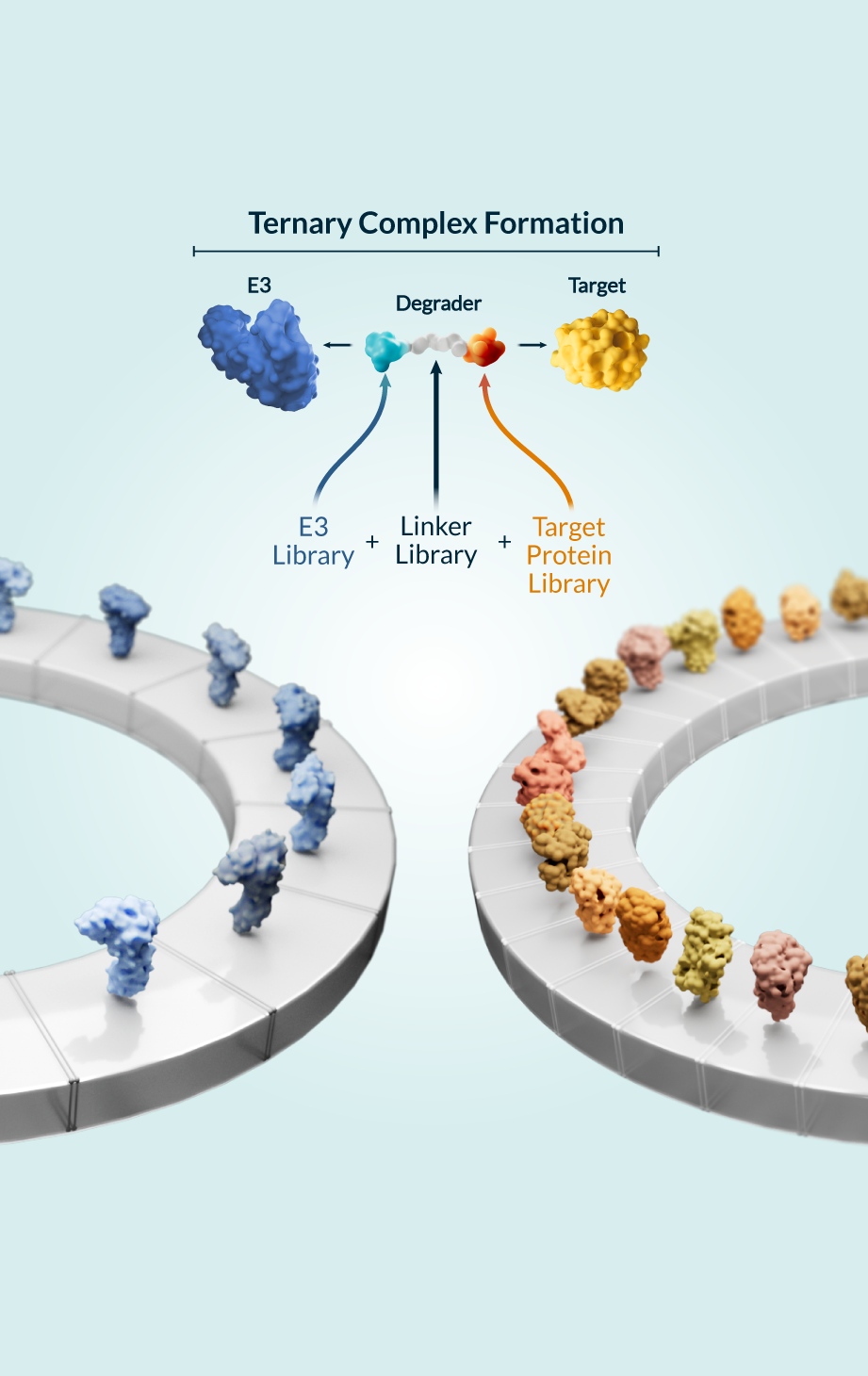
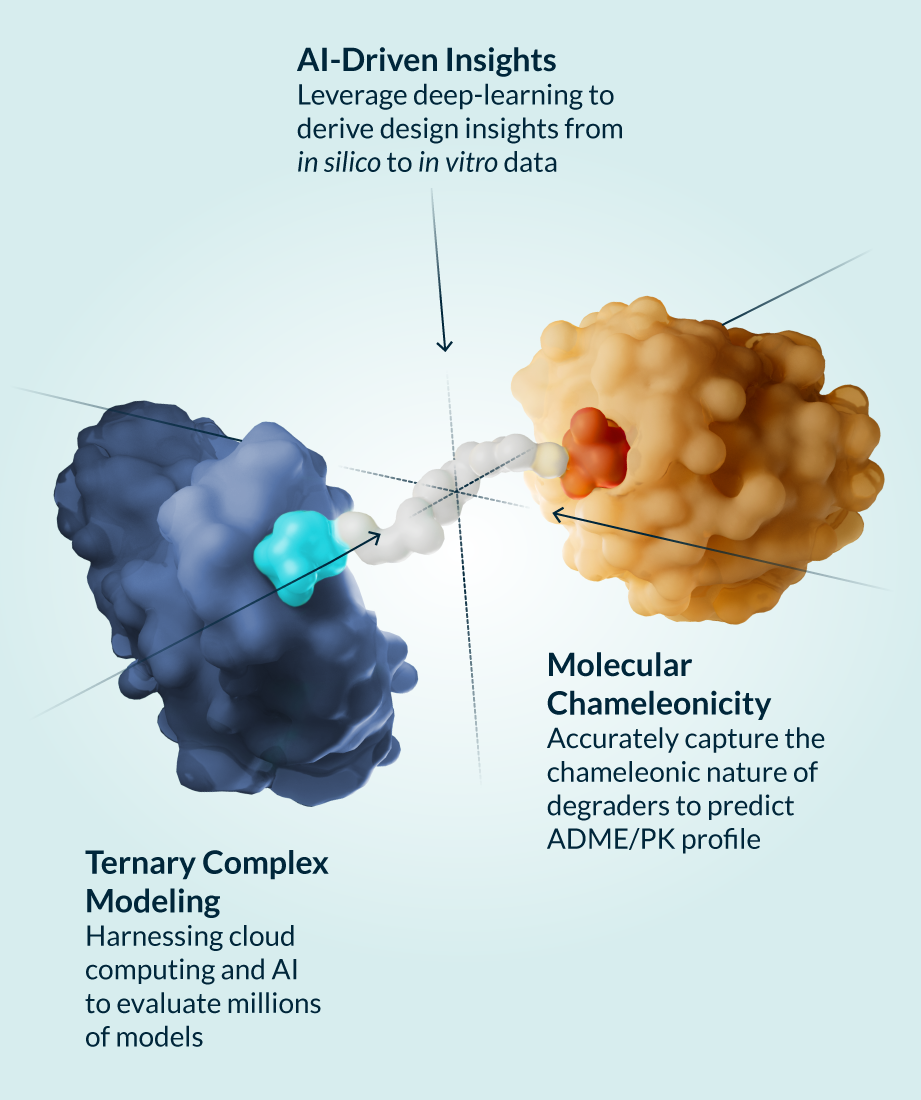
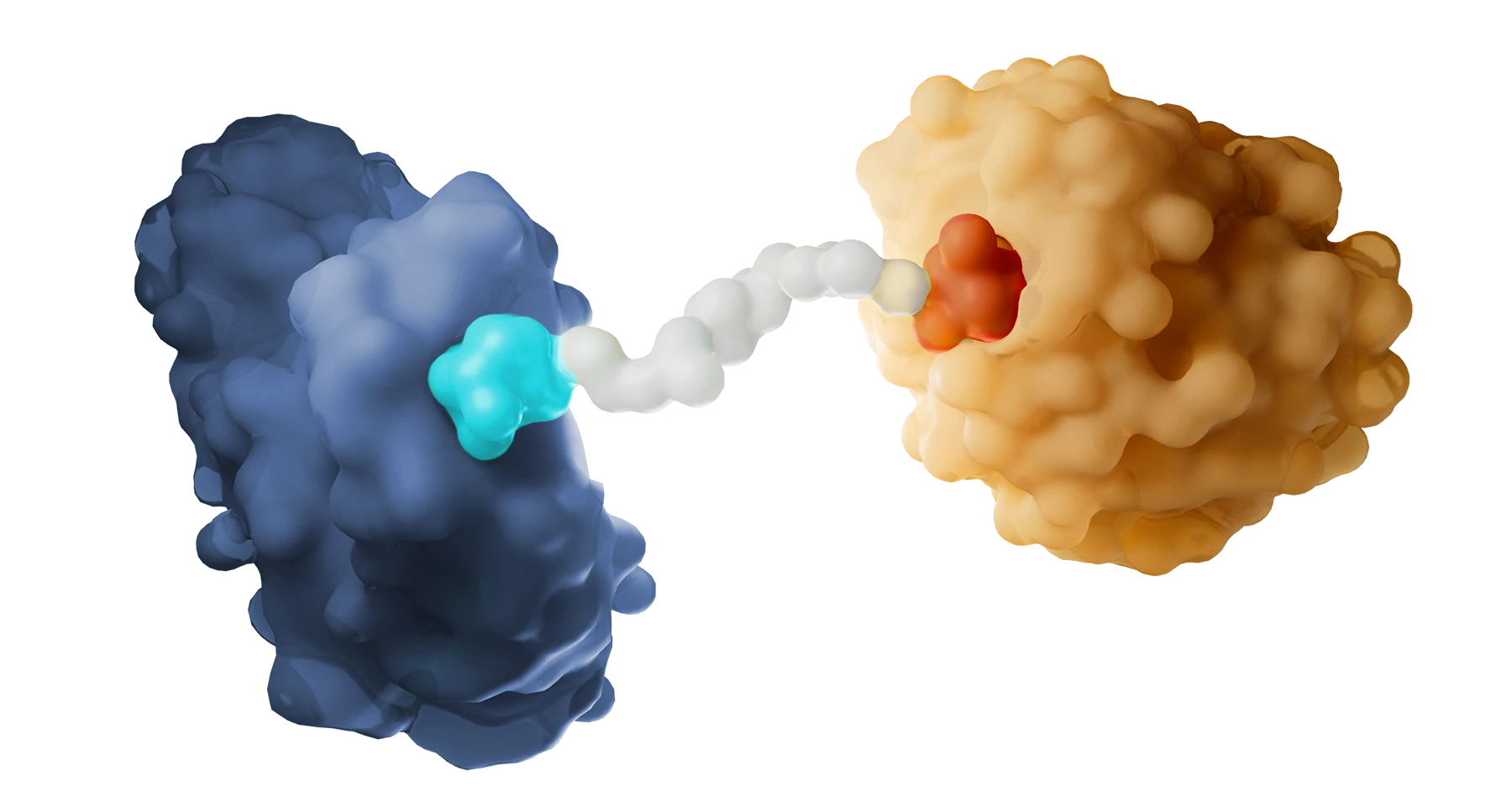
With this promising technology, we have the potential to overcome the inherent limitations of other modalities and can design medicines that address diseases that have long been out of reach, including those associated with undruggable and hard-to-target proteins. And because our molecules can run this same degradation process over and over, we can deliver therapies that potentially require less frequent dosing, which may result in an improved safety profile.
TPD therapies are the kind of big idea that’s upending traditional wisdom about how drugs need to work—and opening doors many people never thought possible.

We’re building a fully integrated biopharmaceutical company focused on meaningfully improving patient care through our unique nexus of biology, proprietary chemistry, data science, and translational research approaches. Our programs address well-validated and high value disease targets and broader biological pathways in therapeutic areas affecting large numbers of patients, where TPD is the best or only option to transform the standard of care.
We strive to achieve what may have seemed impossible in solving critical health problems.
We advanced the first oral small molecule degrader into the clinic for immunological diseases, and we’re progressing degrader medicines in oncology that target undrugged or poorly drugged proteins to create new ways to fight cancer that can potentially treat both solid and liquid tumors.
We continue to drive our science forward and evolve TPD and adjacent technologies by employing cutting-edge computational and research tools to enhance our understanding of the fundamental biological mechanisms of disease to build a deep and broad pipeline.

Get More Out of Life
We strive to give our people and their families benefit offerings that go above and beyond the physical to support your emotional and financial wellbeing. We offer competitive programs that enable employees to better integrate work, family, and community in a healthy, meaningful way and make it easy for you to enjoy your journey at Kymera. These programs will ensure you are supported, create opportunities for growth, and better connect you to our Kymera community.
Need a break? We’ve got you covered. With three office shutdowns, summer hours, and paid time off, we want to give you the flexibility and time you need to step away from your work when you need it most. Our teams also get energy from engaging in philanthropic efforts and interacting socially, and we create ongoing opportunities to give back to our community and do fun things together—everything from knitting clubs to pickleball!
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Vivamus sagittis lacus vel augue laoreet rutrum faucibus dolor auctor. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Fusce dapibus, tellus ac cursus commodo, tortor mauris condimentum nibh, ut fermentum massa justo sit amet risus. Duis mollis, est non commodo luctus, nisi erat porttitor ligula, eget lacinia odio sem nec elit. Nullam id dolor id nibh ultricies vehicula ut id elit. Fusce dapibus, tellus ac cursus commodo, tortor mauris condimentum nibh, ut fermentum massa justo sit amet risus.
Donec id elit non mi porta gravida at eget metus. Nullam quis risus eget urna mollis ornare vel eu leo. Cras justo odio, dapibus ac facilisis in, egestas eget quam. Praesent commodo cursus magna, vel scelerisque nisl consectetur et. Aenean lacinia bibendum nulla sed consectetur. Etiam porta sem malesuada magna mollis euismod.
Donec sed odio dui. Nulla vitae elit libero, a pharetra augue. Maecenas sed diam eget risus varius blandit sit amet non magna. Aenean eu leo quam. Pellentesque ornare sem lacinia quam venenatis vestibulum. Etiam porta sem malesuada magna mollis euismod. Duis mollis, est non commodo luctus, nisi erat porttitor ligula, eget lacinia odio sem nec elit.
Management Team







Board of Directors
Kymera’s pipeline is focused on addressing disease targets where there is significant patient need and where we believe protein degradation is the only or best way to improve the standard of care.
KT-474 is a potential first-in-class IRAK4 degrader in development for the treatment of immune-inflammatory diseases with significant patient need, such as hidradenitis suppurativa (HS), atopic dermatitis (AD), and potentially others. IRAK4 is a key protein of the myddosome complex that mediates signaling through IL-1 and toll-like receptors, which play a crucial role in initiating the immune response against invading pathogens. IRAK4 is a kinase that acts at the interface of the innate and adaptive immune responses with a variety of functions depending on its kinase activity and scaffolding function. Eliminating IRAK4 completely through degradation impacts both the kinase and scaffolding functions, therefore has the potential to achieve a broad, well-tolerated, anti-inflammatory effect providing a novel therapeutic approach.
Atopic Dermatitis
Hidradenitis Suppurativa
Choose an Area of Focus
We are addressing the root molecular causes of chronic inflammatory disease.
We are generating new knowledge on how to apply TPD to both liquid and solid tumors.
Discover more about our cutting-edge toolbox of integrated approaches to accelerate the discovery and development of transformative medicines.
Our leadership in TPD is propelling novel medicines forward.
Featured Perspectives



Nature Reviews Drug Discovery: Protein Degraders Push into Novel Target Space
Our CEO, Nello Mainolfi, was interviewed by Nature Reviews Drug Discovery to discuss how targeted protein degradation can unlock previously intractable disease targets, including our STAT6 and IRAK4 degraders in immunology.
PharmaVoice 100 Top Industry Leaders: Dr. Jared Gollob, Chief Medical Officer
A physician by training, Dr. Jared Gollob’s biopharma career has been rooted in the belief that patients’ needs come first. And it’s one he carries with him as chief medical officer of Kymera Therapeutics, a clinical-stage biotech targeting diseases once considered undruggable.
Dermatology Times: Study Highlights KT-474’s Effectiveness in Targeting IRAK4 for Hidradenitis Suppurativa and Other Inflammatory Diseases
Dermatology Times spoke with Jared Gollob, MD, Chief Medical Officer of Kymera Therapeutics, to discuss the role of IRAK4 expression and degradation in HS, as well as the potential of KT-474 in immuno-inflammatory diseases.
BiotechTV: Nello Mainolfi Shares an Update on Kymera and the Progress of the Protein Degradation Field
Nello gives updates on programs such as IRAK4, STAT6, TYK2, and STAT3, and describes learnings in protein degradation that Kymera has discovered since the last time BiotechTV visited the company one year ago.
Drug Target Review: Women in STEM with Juliet Williams
As part of their series highlighting women in STEM, Drug Target Review spoke to Juliet Williams, Head of Research at Kymera. She discusses what inspired her early passion for science, the power of mentorship and what excites her most about the future of targeted protein degradation – and its potential to deliver results in previously undruggable conditions.
PharmaLeaders: Upending Traditional Wisdom at DDW, an Interview with Nello Mainolfi, PhD
PharmaLeaders had the chance to discuss Kymera’s exciting progress with Founder, President and CEO, Nello Mainolfi, Ph.D.
Boston Business Journal: Biotech Upgrades to New Watertown Digs with Triple the Space
The Boston Business Journal highlighted Kymera’s recent move to enable the expansion of our innovative R&D capabilities and support our growing team’s vibrant on-site presence as we work toward delivering life-changing medicines to patients.
Scrip: Kymera Puts Emphasis On Immunology In New Growth Phase
CEO Nello Mainolfi talked to Scrip about Kymera’s pipeline expansion, which will prioritize oral targeted protein degraders in immunology, including two new assets moving into the clinic.
Endpoints News: Kymera doubles down on protein degraders for immunology, chasing Dupixent and Sotyktu
Looking to further expand the reach of protein degraders beyond cancer, Kymera Therapeutics revealed two new programs Thursday morning that it hopes will compete with some of the hottest immunology drugs on the market.
Genetic Engineering & Biotechnology News: Up-to-$2B Sanofi Collaboration Pays Off for Kymera
Through a pair of recent clinical milestones, Kymera Therapeutics continues to cash in on its up-to-$2 billion collaboration with Sanofi to develop first-in-class targeted protein degradation (TPD) therapies for patients with immune-inflammatory diseases.
KT-621 is an investigational degrader in development for the treatment of immunological and inflammatory diseases
The Phase 1 trial will evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of orally administered KT-621 in healthy volunteers. The study includes double-blind, placebo-controlled single ascending dose (SAD) and multiple ascending dose (MAD) cohorts.
OPEN
KT-474 (SAR444656) is an investigational degrader in development for the treatment of immunological and inflammatory diseases
The safety and efficacy of KT-474 (SAR444656) is currently being evaluated in double blind, placebo-controlled, randomized Phase 2 clinical trials in adult patients with moderate to severe Hidradenitis Suppurativa and Atopic Dermatitis.
OPEN
KT-253 is an investigational degrader in development for the treatment of solid tumors and hematological malignancies
This is an open-label Phase 1 first-in-human study of KT-253 in adult patients to evaluate safety, tolerability, pharmacokinetics/pharmacodynamics, and clinical activity in liquid and solid tumors.
ACTIVE, NOT RECRUITING
KT-333 is an investigational degrader in development for the treatment of solid tumors and hematological malignancies
This is an open-label Phase 1 first-in-human study of KT-333 in adult patients to evaluate safety, tolerability, pharmacokinetics/pharmacodynamics, and clinical activity in liquid and solid tumors.
ACTIVE, NOT RECRUITING

