
Reinventing Medicine
with Protein Degradation
Expanding the Range of
Treatable Disease
Kymera is committed to advancing cutting-edge science to develop a new generation of medicines that address critical health problems and dramatically improve patients’ lives by targeting the fundamental drivers of disease.

Targeted protein degradation is a disease-agnostic modality fundamentally changing how drugs can work, creating unprecedented opportunities to treat a wide variety of diseases.

Our team is committed to delivering life-changing medicines with passion, focus, and a sense of urgency.
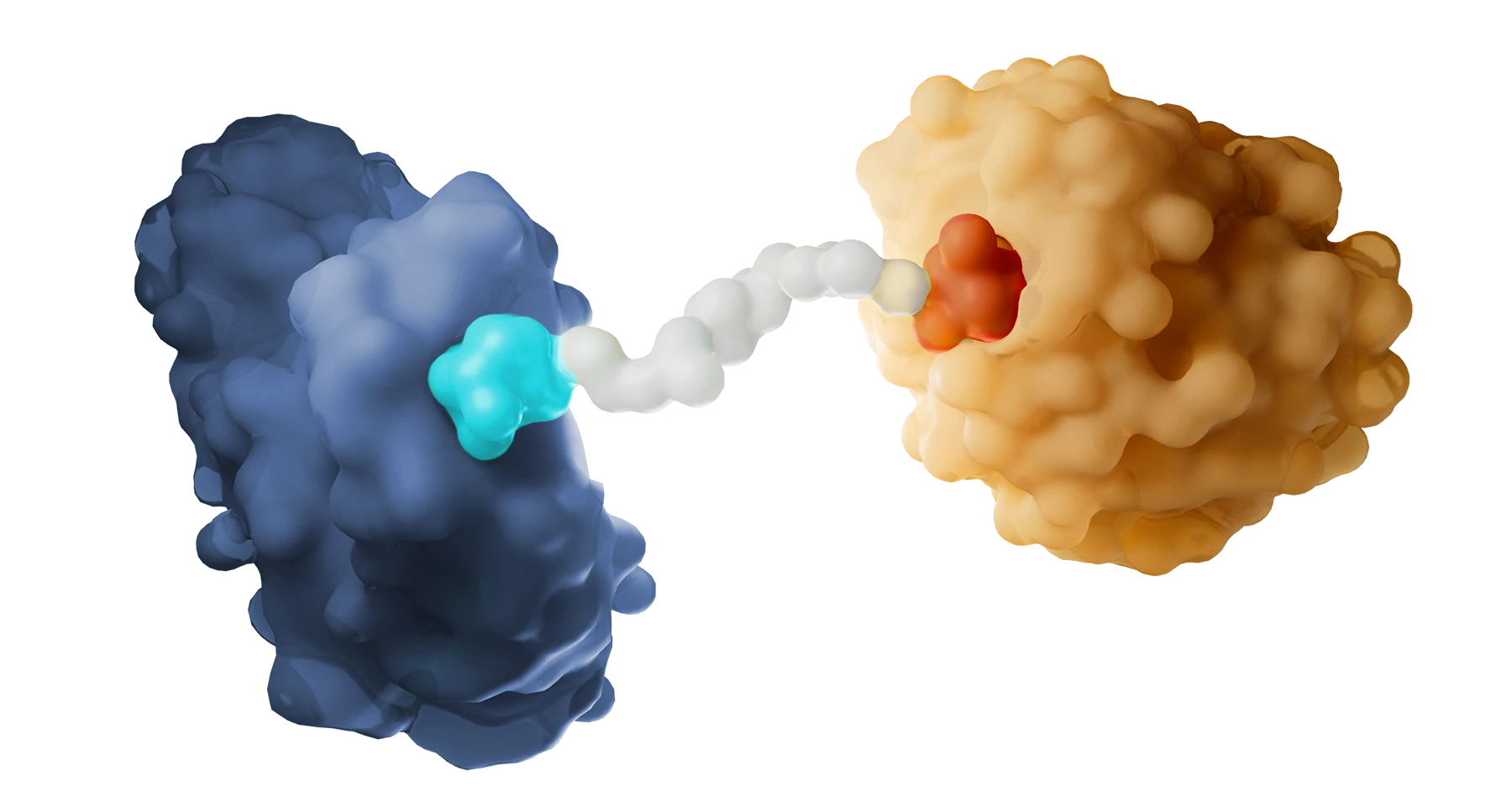
Driven by our powerful and highly productive drug discovery engine, we are delivering a pipeline of first-in-class therapeutics designed to transform the standard of care for patients.


Kymera is building a fully integrated medicines company to redefine disease treatment. Learn more about how you can help shape the future of medicine.